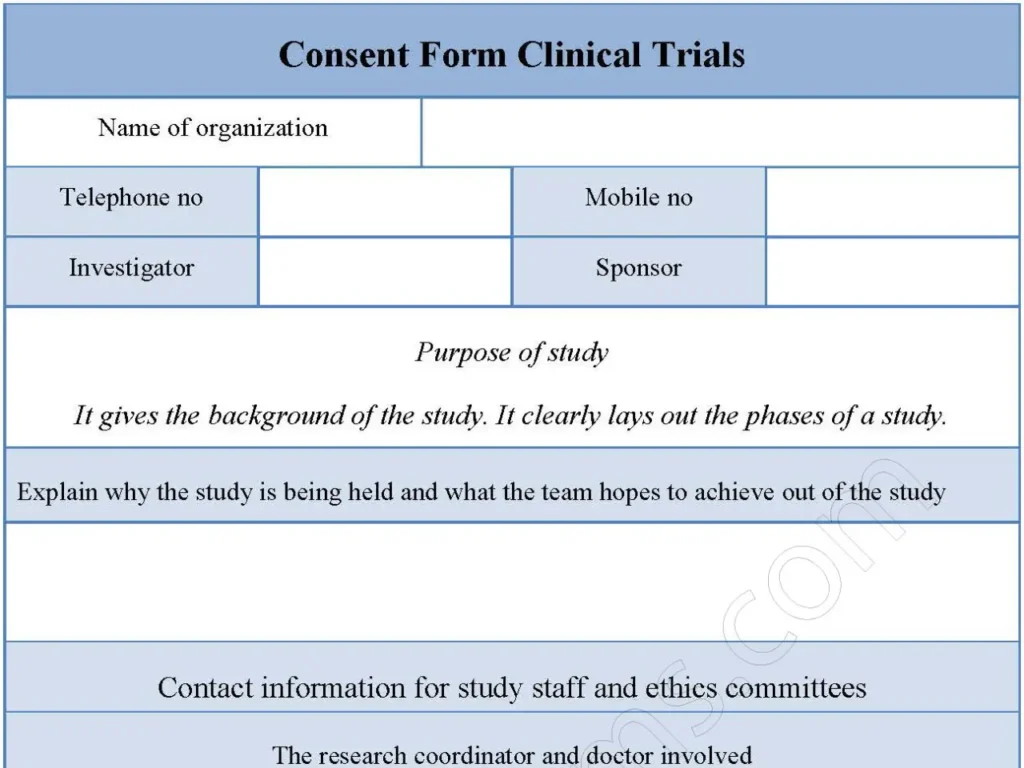
A study volunteer participating in a clinical trial exercise fills out a consent form for clinical trials. Though it is always bulky in size, the information is usually in three parts: the study’s purpose, the study staff contacts, and the volunteer’s rights. Below is a sample consent form for clinical trials.
You can download the Consent Clinical Trials Form Template, customize it according to your needs, and Print it. The consent Clinical Trials Forms Template is in MS Word or Editable PDF.
Download the Consent Form Clinical Trials Form Template for only $6.54.
Buy Now: 6.54 USDIf you are having problems downloading a purchased form, don’t hesitate to contact us and include your receipt number and the exact name of the document you purchased, and I’ll email you a copy.

Features
Clear and concise layout:
The template presents information in a straightforward manner, using simple language and a clear structure.
Essential information:
It includes crucial details such as the name of the organization, contact information, investigator, sponsor, and purpose of the study.
Participant rights:
The template outlines the participant’s rights, including information about the benefits and risks of the study.
Safety measures:
It addresses how the safety of the participant will be protected during the clinical trial.
Consent and signatures:
The template includes sections for the participant and doctor to sign and date the form, indicating agreement to participate in the study.
Benefits
Streamlined process:
The template provides a standardized format for obtaining informed consent, saving time and reducing errors.
Clear communication:
The template facilitates clear communication between the researcher and the participant about the study’s purpose, risks, and benefits.
Legal protection:
The template helps ensure compliance with legal and ethical standards for conducting clinical trials.
Participant empowerment:
By outlining participant rights and safety measures, the template empowers individuals to make informed decisions about their participation.